They cannot look out far.
Robert Frost, “Neither Out Far Nor In Deep”
They cannot look in deep.
But when was that ever a bar
To any watch they keep?
In 2007, Mr. O’Shea1 presented to my clinic, aged 56, after he couldn’t figure out a golf score. The way the numbers lined up no longer made sense. He tailgated a truck, telling his distressed wife he had to center himself on the road by following a close target. The eye doctors told him everything was normal, then he came to see me. When I showed him a photo of a person in a hammock, he pointed at the edge, “string.” He could only see the small parts, not the whole, not even the person.
His was a classic case of Posterior Cortical Atrophy (PCA). Dr. Oliver Sacks reported the same disease as “The Man Who Mistook His Wife for a Hat.” Now we know it as a type of Alzheimer’s disease that first affects visual association areas. Like the typical variant that presents with memory loss, it is progressive and fatal.
Mr. O’Shea got into a clinical trial investigating a drug called bapineuzumab, bapi for short. Bapi was a man-made antibody against β-amyloid, aiming to clear it from the brain. Bapi eventually showed that it slightly reduced β-amyloid accumulation, but it had no clinical benefit.2
Since then, many thousands enrolled in AD trials, each one put their lives on the line. Thanks to them we’ve made progress. We’ve learned a lot about amyloid: right targets, right dose, and right subjects.
On June 7, one of these amyloid drugs, aducanumab, ‘adu’ for short, got an ‘accelerated’ approval. It should have marked the start of human victory against a dread disease. We need to go back a bit to understand where we find ourselves now: a place of acrimony and uncertainty. Biogen conducted two identical trials, twins. In early 2019 a safety board halted both, finding that the combined trials had a low chance of being positive. But when every trial subject’s final visit was tallied and analyzed, something wondrous happened: one trial was positive, one trial was negative. One twin blessed, one cursed. Biogen submitted this data to the FDA for approval.
Like the twin studies themselves, two opposite camps emerged: a skeptical majority, an enthusiastic minority, with very few in the middle. One skeptic wrote an article saying that he would never prescribe the drug if it was approved. While rigid, it is understandable, the drug causes brain swelling in 35% of the subjects. Here’s what that looks like on MRI (red circle):3
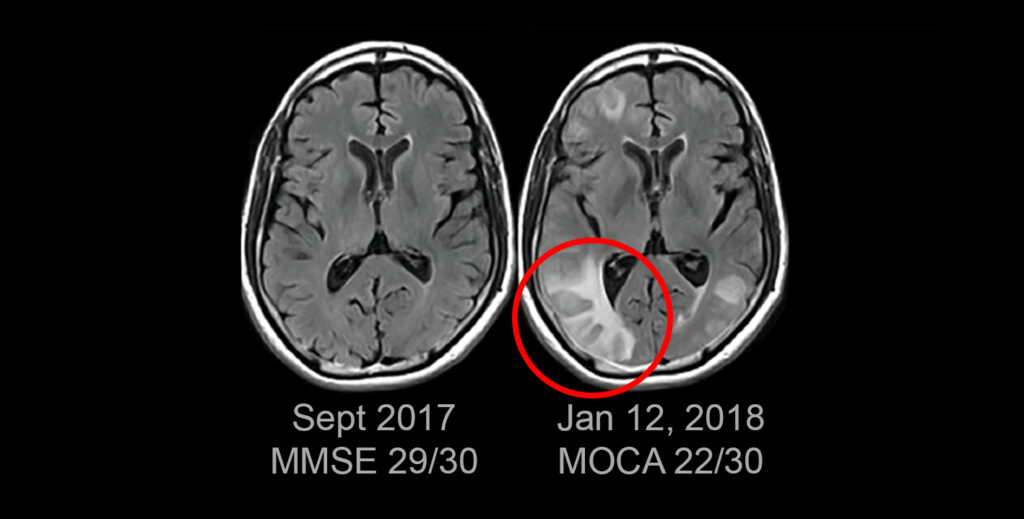
This event fully resolved, but left the subject in a medical ICU with headache, confusion, and high blood pressure.
The FDA convened a meeting with 11 independent experts with backgrounds in medicine, policy, law, and biostatistics. Biogen made their case but the facts remained: one trial positive, one negative. The FDA advisory panel grew frustrated. After Biogen was done, Dr. Scott Emerson, a biostatistician at the University of Washington said this:
SCOTT EMERSON: This analysis seems to be subject to the Texas sharpshooter fallacy, a name for the joke of someone first firing a shotgun at a barn and then painting a target around the bullet holes.
The panel disliked the Biogen’s data and the FDA’s tone. A Biogen doctor told the panel that one negative study “does not detract” from a positive study. Dr. Emerson said this was not only incorrect but seemed to be find support “with some complicity from the FDA clinical staff.” The panel voted against approval, without a single yes vote:
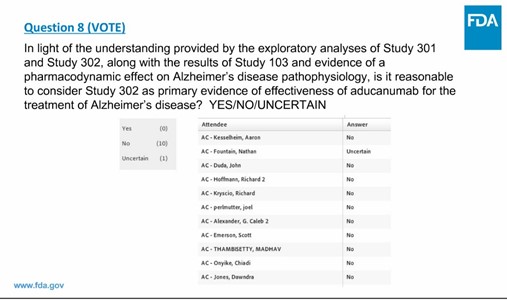
About six months later the FDA approved aducanumab via the Accelerated Approval Program. This permitted aducanumab’s removal of amyloid, and not clinical effect, as allowing aducanumab on the market. Because clinical effectiveness is not established, Biogen must complete another trial showing effect by 2030. Three advisors resigned in protest.
A conditional approval: the drug gets rid of brain amyloid as measured on PET scans. As to clinical benefit, we just don’t know, one twin positive, one negative. We are about to go live with uncertainty.
Bapi also removed amyloid to a limited extent, but it did Mr. O’Shea no good. Many predict a clinical benefit with adu’s robust removal of amyloid (I’m even one of them). But that prediction is not certain. The FDA seems to be pointing to one trial and ignoring the whole. Pointing to the hammock “string.”
“Never treat the scan, treat the person” my mentors told me, a hundred times, then a thousand, tattooed in my mind. And now, at the most delicate tipping point, a semi-approval based on scans, not on people.
The first airplane was unsafe and ugly. We wouldn’t put our family members onboard. We now have a first drug to treat Alzheimer’s disease and there are glimmers it got off the ground.
In a letter to the advisory panel, FDA’s Dr. Dunn doesn’t quite know how to handle this, “Ultimately, the decision on whether aducanumab will be used for treatment will be made by patients, their families and caregivers, and health care professionals.”
It is appealing to be pure minded and restrict this drug to trials. It is also tempting to adopt a false certainty and state the drug works. Amyloid is toxic and causes Alzheimer’s, and removing it is good. But we humans do not see out far nor in deep. We must balance the data with the real people suffering from Alzheimer’s disease. Adopting a hard skepticism disallows autonomy. The skeptics ignore a single positive study, which feels like truth: the drug may work. And yet, an evil twin lurks on the other side: risk of brain swelling with unknown benefit. Do no harm.
While we might not have clear progress, we do have an advance. A staggering step forward is better than one backwards blow. Only three years ago it was hard to imagine removing amyloid at all. Now we can see an amyloid PET scan turning negative, and in one trial we see a slowing of progression in a dread disease. How do we balance these opposing mandates outside the safety of a clinical trial? Where to go from here? In the long run, we will continue to develop more drugs, more certainty, and reduce suffering. In the coming months and years, we must bring incomplete and contradictory information to patients as best we can.
- Details changed to de-identify and protect privacy.
- Liu E, Schmidt ME, Margolin R, et al. Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85(8):692-700.
- VandeVrede L, Gibbs DM, Koestler M, et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab Alzheimers Dement (Amst). 2020;12(1):e12101. Published 2020 Oct 9. doi:10.1002/dad2.12101